Origin of life
Article curated by Joshua Fleming
How did life begin? One of the most basic and yet deeply complex questions ever asked has been met with a multitude of suggestions ranging from religion to science and everything in between, without there ever being conclusive evidence being produced. In this article we’ll explore some key scientific theories, involving everything from planets to proteins.
4 billion years ago our planet was, unsurprisingly, quite a different place. Volcanoes were forming and erupting and asteroids were regularly smashing into the surface, gradually shaping the Earth’s surface. A chaotic, volatile and unlikely place for life to begin. But within “just” several hundred million years, begin it did. The earliest chemofossil - a fossil made of the chemicals left after decomposition of the original organism - dates back to around 3.8 billion years ago. So how did it happen? Well, we can look at how it could have happened, but we may never know for sure. Scientists hope that a ‘true’ understanding of the field, as well as progress in artificial biologies could be gleaned from the comprehension of how life first began.

There are two main categories of hypotheses being proposed for the origin of life, replicator-first, or metabolism-first. Metabolism-first hypothesis focuses on simple molecules that could have gradually developed into the complex compounds for life. Conversely, the Replicator first hypothesis is comprised of the idea that naturally occurring molecules could already self replicate and life grew from these instead.
To understand how a living organism is built, you first have to know about the blocks required to build it. There are three fundamental compounds found in everything alive; nucleic acids, proteins and lipids. The important nucleic acids for life are deoxyribonucleic acid and ribonucleic acid. They sound complicated but are usually referred to as DNA and RNA, terms you may have come across before. The proteins are effectively the builders, folding into shapes specific to their job within the cell. Finally, lipids are responsible for the general structure of the cell - keeping all of its components inside the membrane. Without these molecules cells wouldn’t exist; life wouldn’t exist.

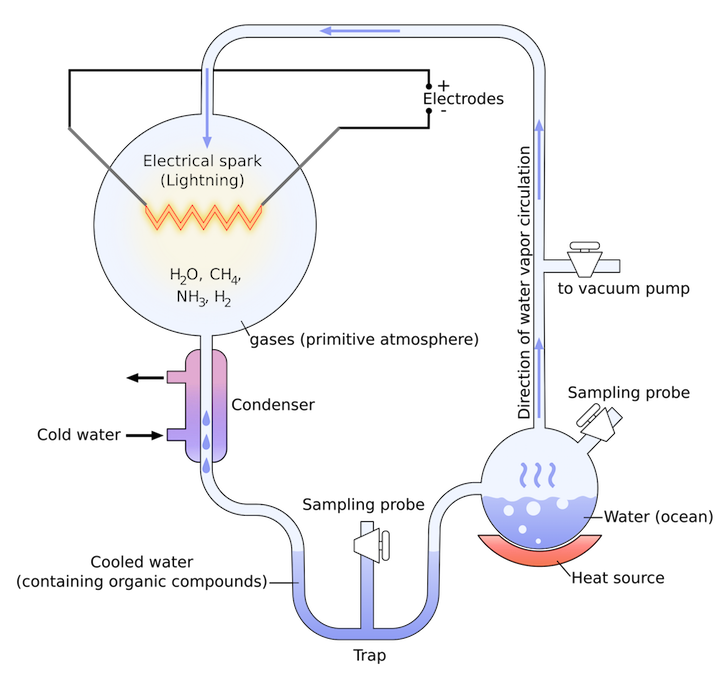
In 1958, the famous researcher Stanley Miller carried out an experiment [1]. Little did he know it would lead to some of the strongest evidence that supports a replicator-first theory. He had previously theorized that the atmosphere of the Earth billions of years ago contained a combination of the perfect elements and compounds for life to begin, including hydrogen, water, ammonia and methane. These building blocks could join to form amino acids (which are a step between RNA and proteins) and sugars, the precursors to life. All that was needed for this to happen was a spark (from lightning for example) hence its name the ‘electric-spark’ hypothesis. Miller tested this in 1953 with another scientist, Harold Urey, and so it was named the Miller-Urey experiment. The resulting samples produced did contain amino acids, but only five.
When Miller died in 2007 one of his former students (Jeffrey Bader) received some of his old experimental samples as an inheritance of sorts.These samples had come from a very similar test that Miller did half a decade after 1953, but for an unknown reason they weren’t analysed at the time. When Bader (among others) began exploring the products, he discovered an astonishing amount of amino acids had been produced. The key difference in this experiment to the one in 1953 was that Miller had added one more component to the atmospheric mix; hydrogen sulphide (H2S). Volcanoes naturally release this gas and so it became conceivable that instead of the elements of life forming in atmospheric clouds with lightning, perhaps life had been ‘sparked’ in a volcanic cloud.

Shapiro is among a lot of scientists that do not agree with replicator-first hypotheses. Another major proposition is that life originated in outer space. This theory, ‘Panspermia’, describes the elements required for life moving through our universe hidden on asteroids and meteoroids. If these molecules can survive this transportation, then it is feasible that life could be kickstarted with the energy produced from one collision with our planet.
The scarcity of extant records of this period, coupled with the fact that there would be no clearly identifiable difference observable between rocks acted on by life of terrestrial origin and those acted on by life that had been transported here present us with difficulty. The only way we could say for sure that life had begun elsewhere would be to find a fossilised record of it trapped within an ancient meteorite. Even in this circumstance, it would be impossible to say that life had not begun simultaneously and independently here on Earth.
Until recently there has been little accredited evidence for Panspermia, but a group of researchers have discovered that a combination of different compounds not naturally occurring on early Earth could have shaped RNA [4]. Additionally, RNA is unstable in water - which covered (and still covers) the vast majority of the planet. So where are these compounds found? Mars. The lead scientist of the team, Steve Benner, mentioned that the research was by no means conclusive and wasn’t a definite solution [5].
Learn more about Location of the Origin of Life.


 2
2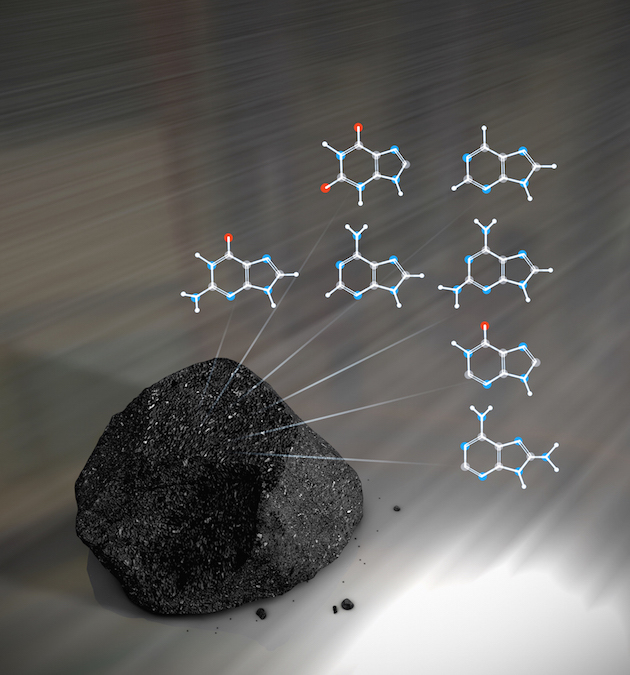
If indeed the building blocks of life came to Earth from outer space, then it should be possible for us to find evidence of these crucial ingredients that are still out there. Glycine is the smallest of the 20 amino acids that most commonly make up proteins, and is a good example of something to seek. A variety of space probes have been designed to look for exactly such evidence - but one major challenge is that it is very hard to distinguish actual interstellar glycine collected by probes from glycine that contaminated the probe before it left Earth.
In 2009, NASA announced that the Stardust mission had detected glycine in material collected from the tail of comet Wild. More recently in 2015, scientists analysing data from the European Philae comet lander reporting finding 16 organic compounds on comet 67P/Churyumov-Gerasimenko, including many nitrogen-bearing species but no sulfur-bearing species, and four compounds—methyl isocyanate, acetone, propionaldehyde, and acetamide—that had not previously been reported in comets
, although the scientists were unable to confirm the presence of glycine as its spectra was difficult to disentangle from the contributions of other species
[6].
Another way of confirming the presence of interstellar glycine is through the analysis of spectral data collected by telescopes, but reported instances of any kind of building block of life, such as amino acids or RNA, have typically been widely disputed.
Learn more about Interstellar glycine.

 2
2One of the main propositions for a replicator-first idea is the ‘RNA-world’ hypothesis. It theorises that RNA was gradually constructed in a mixture of organic molecules that had accumulated on the planet’s surface, known as the ‘primordial soup’. Critics originally argued that the process just couldn’t occur in the projected conditions on early Earth, but in 2009 researchers in Manchester, UK, produced strong evidence that implied otherwise [2]. RNA consists of four subunits, two of which are called purines and the other two, pyrimidines. The team, lead by John Sutherland, managed to induce the synthesis of both of these pyrimidines whilst only using materials that would have been available on Earth at the time. However, some scientists disagree that it reflects the origins of life. A New York University chemist Robert Shapiro responded at the time; The flaw with this kind of research is not in the chemistry. The flaw is in the logic — that this experimental control by researchers in a modern laboratory could have been available on the early Earth,
[3]. Shapiro supports a different hypothesis, based around smaller, less complex molecules than RNA being present first. This ‘simple metabolism’ idea submits that gradually these evolved into cells that could produce the nucleic acids.
Sutherland’s previous project aimed to provide evidence that with the right combination of molecules RNA could be created by itself. However the molecules he used, acetylene and formaldehyde, were too complex to be base molecules and opposition to his research queried where these had come from. His team have now gone one step further (or back in this case) and discovered chemical pathways to RNA from simpler roots. Using only ultraviolet light, hydrogen cyanide (HCN) and the volcanic gas hydrogen sulphide (H2S), the researchers were able to create not only the beginnings of nucleic acids, but the other two crucial building blocks for life as well; proteins and lipids [7]. The research indicated that these reactions still wouldn’t happen all at once, or in one location, but would occur separately and then eventually accumulate. Sutherland is now working on assembling the building blocks his team had created to try to work forwards instead of back. Additionally, as he has only been able to find the routes potentially used for pyrimidines, he hopes to discover a purine formation pathway.


On oceanic seafloors all over Earth there are cracks called hydrothermal vents. Below these is the mantle. Gases including H2S and HCN can travel down through these cracks, before being shot back up with torrid water. Nothing evaporates because of the huge pressures at the depths of the fissures. In fact, the gases then dissolve into the heated water around them, creating a biological mixture ideal for the beginnings of life. The iron-sulphur world hypothesis is a metabolism-first proposition, and suggests that the first simple molecules were created from this mixture when it flowed over various metals in the rocks surrounding the deep sea vents. Iron and other minerals could have acted as catalysts, fast-forwarding the chemical reactions needed to yield the first simple compounds of life. These could then have been coupled with different metals, creating new aggregates more likely to form yet more complex products.

A hypothesis linked to the hydrothermal vents describes the oceans 4 billion years ago as frozen nearly a third of a kilometre thick, due to a less luminous sun. Ultraviolet light would’ve still been at deadly levels without an ozone layer as protection, but a dense layer of ice may have worked just as well. A cool, stable marine environment might have been ideal for the organic molecules provided by the vents. Additionally there would be a massively reduced threat from the effects of impact frustration.

When did life begin?
It is generally accepted that the Earth formed some 4.54 billion years ago, and that in its earliest days the planet was too inhospitable for life. But when exactly did life emerge? In 2015, UCLA geochemists found evidence that life likely existed on Earth at least 4.1 billion years ago — 300 million years earlier than previous research suggested.
- but is this the final answer?
The scientists themselves (rather than the resulting press release and media articles) state that this research simply raises the possibility that biologic processes were operating during the Hadean
, and noted that not all light carbon isotopic signals are biogenic.
[8]
According to their paper, in the absence of experimental evidence for particulate carbon formation via a Fischer–Tropsch process[9][10] or diffusive or mineral disproportionation mechanisms that could selectively lead to light carbon condensates that were ultimately incorporated in a low-temperature (i.e., ∼650 °C) granitoid zircon, a biogenic origin seems at least as plausible. - hinting at possible areas of future research that would help us to better understand these findings.

Is the Earth unique?
Ganymede, Jupiter's largest moon, may actually be composed of several layers of salty waters and ices - much like a club sandwich. Because of the huge pressures thought to exist on Ganymede's ocean floor, scientists had previously believed that the layer next to it's rocky core would most likely be ice. This was a problem for the idea that primitive life may have formed on Ganymede at some point in it's history.
Rock/liquid water interfaces important for the potential development of life - many scientists believe that life on Earth most likely began at hydrothermal vents on our own ocean floor. The findings from a NASA study of Ganymede in 2014 imply that instead of the core being next to water - because of the high salinity, denser fluid water would sink toward the core in Ganymede allowing water and rocks to interact.
Ganymede's impressive ocean was first confirmed to exist when NASA's Galileo mission flew by the moon, revealing the water to be hundreds of miles deep. If scientists add salt into the equation they find that the water becomes denser and sinks. This is due to the attraction of water molecules to salt molecules. By using computerised simulations and models, scientists have concluded that a likely composition of Ganymede's ocean is water sandwiched between up to 3 ice layers, with a salty liquid water layer next to the rocky seafloor.
Steve Vance of NASA's Jet Propulsion Laboratory in California and his team are amongst those looking for evidence that this model might be correct. Moreover, it is not clear how long the satellite's structure would be able to hold this state. Currently the model suggests that Ganymede would be stable like this, however there are many factors which could affect it meaning it never gets to this stage.
Studying the oceans of places like Ganymede could reveal more about how life can exist and teach us more about what clues we can look for in the search for alien life.
Learn more about The composition of Ganymede.

 2
2Maybe we’re from a volcano. Maybe we’re from Mars. Maybe we’re from a volcano on Mars… The only thing we know for certain is that this is something we don’t, and may not ever, know.
This article was written by the Things We Don’t Know editorial team, with contributions from Ed Trollope, Jon Cheyne, Freya Leask, Cait Percy, Grace Mason-Jarrett, and Joshua Fleming.
This article was first published on 2015-09-04 and was last updated on 2018-01-21.
References
why don’t all references have links?
[1] Miller, S. L. 1953. 'A Production Of Amino Acids Under Possible Primitive Earth Conditions'. Science 117 (3046): 528-529 doi:10.1126/science.117.3046.528.
[2] Powner, Matthew W., Béatrice Gerland, and John D. Sutherland. 2009. 'Synthesis Of Activated Pyrimidine Ribonucleotides In Prebiotically Plausible Conditions'. Nature 459 (7244): 239-242 doi:10.1038/nature08013.
[3] Van Noorden, Richard. 2009. 'RNA World Easier To Make'. Nature doi:10.1038/news.2009.471.
[4] Benner, Steven A., Hyo-Joong Kim, and Matthew A. Carrigan. 2012. 'Asphalt, Water, And The Prebiotic Synthesis Of Ribose, Ribonucleosides, And RNA'. Acc. Chem. Res. 45 (12): 2025-2034 doi:10.1021/ar200332w.
[5] Webb, Richard. 2015. 'Primordial Broth Of Life Was A Dry Martian Cup-A-Soup'.
New Scientist
[6] Goesmann, F., et al., (2015) Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry Science 349 (6247) DOI: 10.1126/science.aab0689
[7] Patel, Bhavesh H., Claudia Percivalle, Dougal J. Ritson, Colm D. Duffy, and John D. Sutherland. 2015. 'Common Origins Of RNA, Protein And Lipid Precursors In A Cyanosulfidic Protometabolism'. Nature Chem 7 (4): 301-307 doi:10.1038/nchem.2202.
[8] Bell, E.A., et al., (2015) Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proceedings of the National Academy of Sciences online DOI: 10.1073/pnas.1517557112
[9] McCollom, T.M., (2013) Laboratory simulations of abiotic hydrocarbon formation in Earth’s deep subsurface. Reviews in Mineralogy & Geochemistry 75:467-494 DOI: 10.2138/rmg.2013.75.15
[10] Shilobreeva, S., et al., (2011) Insights into C and H storage in the altered oceanic crust: Results from ODP/IODP Hole 1256D. Geochimica et Cosmochimica Acta 75.9:2237-2255 DOI: 10.1016/j.gca.2010.11.027
Recent origin of life News
Get customised news updates on your homepage by subscribing to articles